
Quadruple Nucleic Acid Test Kit for Influenza Virus (A, B) , Novel Coronavirus, Respiratory Syncytial Virus
Hunan Runmei Gene Technology Co., Ltd.- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Classification:Biological Diagnostics
- Certification:CE, FDA, EEC, MSDS, ISO13485
- Transport Package:Carton
- Specification:58.5*58.8*62.5cm
Base Info
- Model NO.:SKY-82140
- Trademark:Runmei
- Origin:China
- HS Code:1108966068
- Production Capacity:10000boxes,Day
Description
Basic Info.
Model NO. SKY-82140 Trademark Runmei Origin China HS Code 1108966068 Production Capacity 10000boxes/DayProduct Description
Quadruple Nucleic Acid Test Kit for Influenza virus (A, B), Novel Coronavirus, Respiratory Syncytial Virus (Fluorescent RT-PCR method)
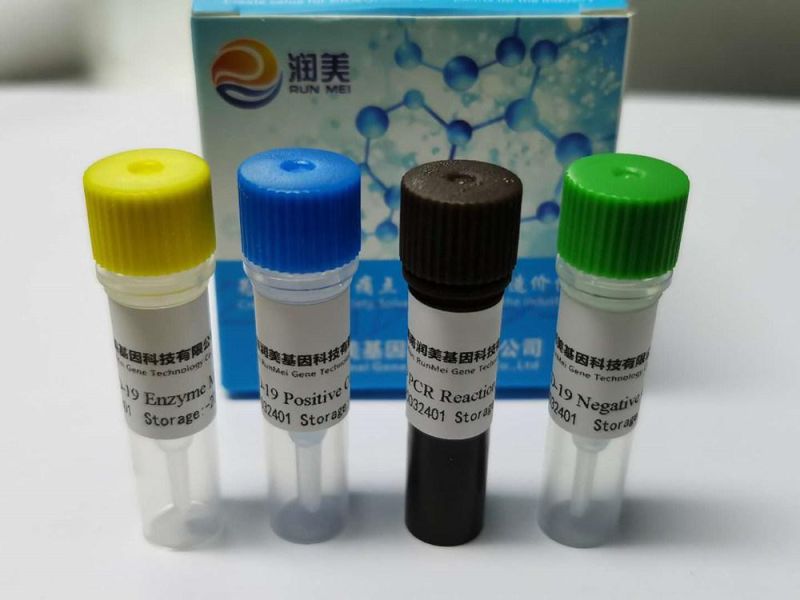

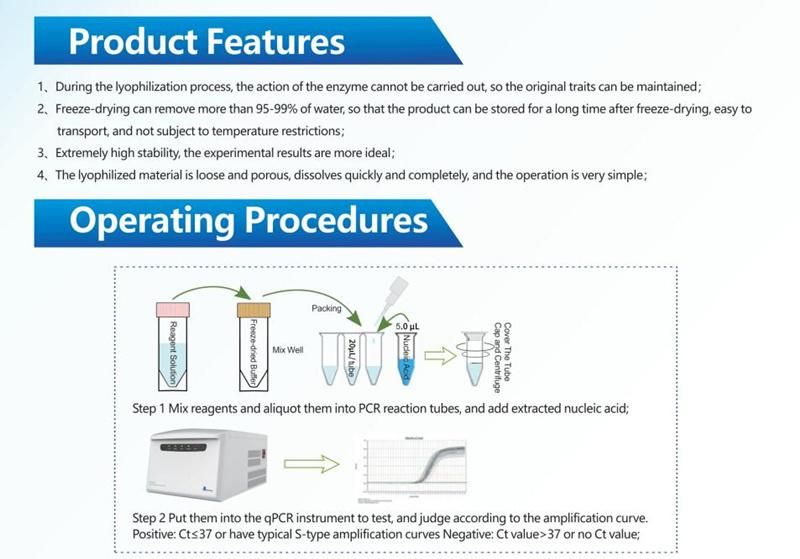

Company Profile:
to base on China and radiate the world, and to solve the pain points and difficulties of the industry and create value for human beings as our corporate purpose. At present, our company has completed the construction of product systems for pathogen biology fluoresence quantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biology immune colloidal gold detection kits,and disposable syringes.







