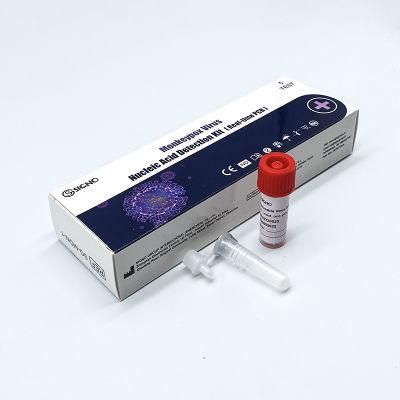
Cure Vaccine Hazmat Product Check Examination Kit Monkeypox
Shenzhen Signo Group Technology Co., Ltd.- Type:Test Strips & Test Tube
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult and Children
- Logo Printing:With Logo Printing
Base Info
- Brand:Signo
- Function:Monkeypox Virus Nucleic Acid Detection
- Name:Monkeypox Virus Test Kits
- High Sensitivity:The Limit of Detection of The Kit Is 200 Copies,Ml
- High Specifity:No Cross-Reactivity with Other Pathogens
- Convenient Detection:67min Amplification
- Transport Package:Carton
- Specification:5 test,box, 25 test,box
- Trademark:Signo
- Origin:China
- Production Capacity:500000 PCS,Day
Description
Cure Vaccine Hazmat Product Check Examination Kit Monkeypox
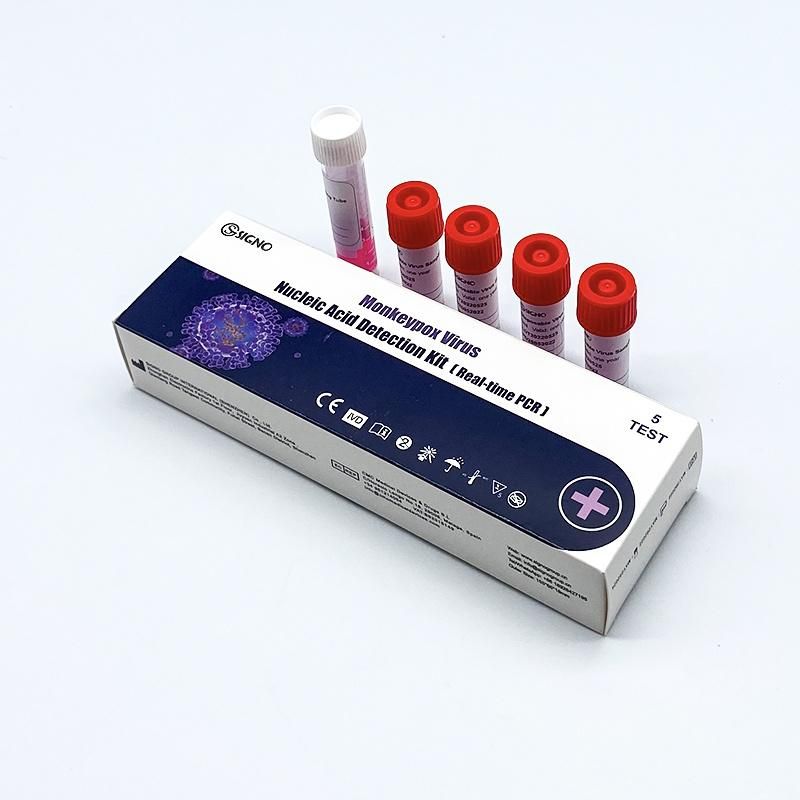
Monkeypox Diagnostic Test Kits Intended Use:
The kit is used for in vitro qualitative detection of suspected cases of Monkeypox Virus (MPV), clustered cases and
other cases that need to be diagnosed for Monkeypox Virus infection.
Monkeypox Virus Description:
Monkeypox is a viral zoonotic disease caused by monkeypox virus. It occurs primarily in tropical rainforest areas of
Central and West Africa and is occasionally exported to other regions. Monkeypox is transmitted to humans through
close contact with an infected person or animal, or with material contaminated with the virus. The incubation period
of monkeypox is usually from 6 to 13 days but can range from 5 to 21 days.
Monkeypox Symptoms:
Fever,Rash,Exhaustion,Swollen lymph nodes.
Test Method:
Real Time Fluorescence PCR Test.
Test Principle:
This kit takes the specific conserved sequence of the MPV f3L gene as the target region. The real-time fluorescence
quantitative PCR technology and nucleic acid rapid release technology are used to monitor the viral nucleic acid through
the change of fluorescence signal of amplification products. The detection system includes internal quality control,
whichis used to monitor whether there are PCR inhibitors in the samples or whether the cells in the samples are taken,
which can effectively prevent the false negative situation. The test results of this kit are for clinical reference only and
should not be used as the sole criterion for clinical diagnosis. It is recommended to conduct a comprehensive analysis
of the condition based on the patient's clinical manifestations and other laboratory tests.
Sample Type:
Whole Blood/ Serum/Plasma/throat swabs/ nasal swabs.
Kits Features:
High sensitivity:The limit of detection of the kit is 200 copies/mL.
Rapid Diagnosis:Test 94 samples at the same time within 70 mins, helping rapid clinical diagnosis.
Reliable Results:Using a quality control system with dUTP enzyme pollution prevention system and internal control
supervise system,the results are more reliable.
High Specifity: No cross-reactivity with other pathogens.
Convenient detection:67min amplification
Main Components:
Instruction:
(1) Sampling solution
After verification, it is recommended to use normal saline or Virus preservation tube for sample collection.
throat swab: wipe bilateral pharyngeal tonsils and posterior pharyngeal wall with disposable sterile sampling swab,
immerse the swab.into the tube containing 3mL sampling solution, discard the tail, and tighten the tube cover.
(2) Sample storage and delivery
The samples to be tested should be tested as soon as possible. The transportation temperature should be kept at
2~8ºC.The samples that can be tested within 24 hours can be stored at 2ºC~8ºC and if the samples cannot be tested
within 24 hours, it should be stored at less than or equal to -70ºC (if there is no storage condition of -70ºC, it can be
stored at -20ºC temporarily), avoid repeated freezing and thawing.
(3) Proper sample collection, storage, and transportation are critical to the performance of this product.
(4) Sample processing
After mixing the above sampling solution with samples, take 30μL of the sample into the DNA release reagent tube
and mix it evenly.
(5) Loading
Take 20μL of the reconstitution reagent and add it to the MPV detection reagent, add 5μL of the above processed
sample (The positive control and negative control shall be processed in parallel with the samples), cover the tube
cap, centrifuge it at 2000rpm for 10 seconds.
(6) PCR amplification
1. Load the prepared PCR plate/tubes to the fluorescence PCR instrument, Negative control and positive control
shall be set for each test;
2. Fluorescent channel setting;
3. Choose FAM channel for MPV detection;
4. Choose HEX/VIC channel for internal control gene detection.
(7) Results analysis
Set the base line above the highest point of the negative control's fluorescent curve.
(8) Quality control
1.Negative control:No Ct value detected in FAM,HEX/VIC channel, or Ct>40;
2. Positive control:In FAM,HEX/VIC channel, Ct≤40;
3. The above requirements should be satisfied in the same experiment, otherwise the test results are invalid and the
experiment needs to be repeated.
[ Cut off value ]
A sample is considered as positive when: Target sequence Ct≤40, The internal control gene Ct≤40.
[ Results interpretation ]
Once the quality control is passed, users should check if there is an amplification curve for each sample in HEX/VIC
channel, if there is and with Ct≤40, it indicated the internal control gene is successfully amplified and this particular
test is valid. Users can proceed to the follow up analysis;
For samples with the amplification of internal control gene failed (HEX/VIC channel, Ct>40,or no amplification curve),
low Viral load or the existence of PCR inhibitor could be the reason of failure, the examination should repeated from
the specimen collection;
For positive samples and cultured virus, the results of internal control do not affect;
For samples tested negative, the internal control needs to be tested positive otherwise the overall result is invalid and
the examination;
needs to be repeated, starting from the specimen collection step.


FAQ:
1, Q: Do you have any certificates for products?
A: Yes, we have many certificates such as CE,ISO13485,FSC.
2, Q: Can I get some samples?
A: Yes, we are glad to offer you some samples for free, but freight shall be collected at your side.
3, Q: how can we guarantee quality?
A: Always a pre-production sample before mass production;Always final Inspection before shipment.
4, Q: What payment term does SIGNO accept?
A: We accept T/T,L/C,Western union and some of other payment methods. Usually we prefer 100% T/T in advance, for special
order, 40% of total amount would be paid when place order and the rest would be paid before shipment.
5, Q: Why choose Signo Group?
A: We are a professional molecular diagnosis company in China. Meanwhile, we provide OEM and ODM service to our clients.


Monkeypox Diagnostic Test Kits Intended Use:
The kit is used for in vitro qualitative detection of suspected cases of Monkeypox Virus (MPV), clustered cases and
other cases that need to be diagnosed for Monkeypox Virus infection.
Monkeypox Virus Description:
Monkeypox is a viral zoonotic disease caused by monkeypox virus. It occurs primarily in tropical rainforest areas of
Central and West Africa and is occasionally exported to other regions. Monkeypox is transmitted to humans through
close contact with an infected person or animal, or with material contaminated with the virus. The incubation period
of monkeypox is usually from 6 to 13 days but can range from 5 to 21 days.
Monkeypox Symptoms:
Fever,Rash,Exhaustion,Swollen lymph nodes.
Test Method:
Real Time Fluorescence PCR Test.
Test Principle:
This kit takes the specific conserved sequence of the MPV f3L gene as the target region. The real-time fluorescence
quantitative PCR technology and nucleic acid rapid release technology are used to monitor the viral nucleic acid through
the change of fluorescence signal of amplification products. The detection system includes internal quality control,
whichis used to monitor whether there are PCR inhibitors in the samples or whether the cells in the samples are taken,
which can effectively prevent the false negative situation. The test results of this kit are for clinical reference only and
should not be used as the sole criterion for clinical diagnosis. It is recommended to conduct a comprehensive analysis
of the condition based on the patient's clinical manifestations and other laboratory tests.
Sample Type:
Whole Blood/ Serum/Plasma/throat swabs/ nasal swabs.
Kits Features:
High sensitivity:The limit of detection of the kit is 200 copies/mL.
Rapid Diagnosis:Test 94 samples at the same time within 70 mins, helping rapid clinical diagnosis.
Reliable Results:Using a quality control system with dUTP enzyme pollution prevention system and internal control
supervise system,the results are more reliable.
High Specifity: No cross-reactivity with other pathogens.
Convenient detection:67min amplification
Main Components:
| Product Name | Monkeypox Diagnostic PCR Test Kit |
| OEM | Yes, support OEM. |
| Packing | 24 Tests/kit, 48 tests/kit |
| Analyte | Monkeypox Virus (MPV) |
| Equipment Required | Any of real-time PCR instruments with FAM and VIC. |
Instruction:
(1) Sampling solution
After verification, it is recommended to use normal saline or Virus preservation tube for sample collection.
throat swab: wipe bilateral pharyngeal tonsils and posterior pharyngeal wall with disposable sterile sampling swab,
immerse the swab.into the tube containing 3mL sampling solution, discard the tail, and tighten the tube cover.
(2) Sample storage and delivery
The samples to be tested should be tested as soon as possible. The transportation temperature should be kept at
2~8ºC.The samples that can be tested within 24 hours can be stored at 2ºC~8ºC and if the samples cannot be tested
within 24 hours, it should be stored at less than or equal to -70ºC (if there is no storage condition of -70ºC, it can be
stored at -20ºC temporarily), avoid repeated freezing and thawing.
(3) Proper sample collection, storage, and transportation are critical to the performance of this product.
(4) Sample processing
After mixing the above sampling solution with samples, take 30μL of the sample into the DNA release reagent tube
and mix it evenly.
(5) Loading
Take 20μL of the reconstitution reagent and add it to the MPV detection reagent, add 5μL of the above processed
sample (The positive control and negative control shall be processed in parallel with the samples), cover the tube
cap, centrifuge it at 2000rpm for 10 seconds.
(6) PCR amplification
1. Load the prepared PCR plate/tubes to the fluorescence PCR instrument, Negative control and positive control
shall be set for each test;
2. Fluorescent channel setting;
3. Choose FAM channel for MPV detection;
4. Choose HEX/VIC channel for internal control gene detection.
(7) Results analysis
Set the base line above the highest point of the negative control's fluorescent curve.
(8) Quality control
1.Negative control:No Ct value detected in FAM,HEX/VIC channel, or Ct>40;
2. Positive control:In FAM,HEX/VIC channel, Ct≤40;
3. The above requirements should be satisfied in the same experiment, otherwise the test results are invalid and the
experiment needs to be repeated.
[ Cut off value ]
A sample is considered as positive when: Target sequence Ct≤40, The internal control gene Ct≤40.
[ Results interpretation ]
Once the quality control is passed, users should check if there is an amplification curve for each sample in HEX/VIC
channel, if there is and with Ct≤40, it indicated the internal control gene is successfully amplified and this particular
test is valid. Users can proceed to the follow up analysis;
For samples with the amplification of internal control gene failed (HEX/VIC channel, Ct>40,or no amplification curve),
low Viral load or the existence of PCR inhibitor could be the reason of failure, the examination should repeated from
the specimen collection;
For positive samples and cultured virus, the results of internal control do not affect;
For samples tested negative, the internal control needs to be tested positive otherwise the overall result is invalid and
the examination;
needs to be repeated, starting from the specimen collection step.



FAQ:
1, Q: Do you have any certificates for products?
A: Yes, we have many certificates such as CE,ISO13485,FSC.
2, Q: Can I get some samples?
A: Yes, we are glad to offer you some samples for free, but freight shall be collected at your side.
3, Q: how can we guarantee quality?
A: Always a pre-production sample before mass production;Always final Inspection before shipment.
4, Q: What payment term does SIGNO accept?
A: We accept T/T,L/C,Western union and some of other payment methods. Usually we prefer 100% T/T in advance, for special
order, 40% of total amount would be paid when place order and the rest would be paid before shipment.
5, Q: Why choose Signo Group?
A: We are a professional molecular diagnosis company in China. Meanwhile, we provide OEM and ODM service to our clients.
