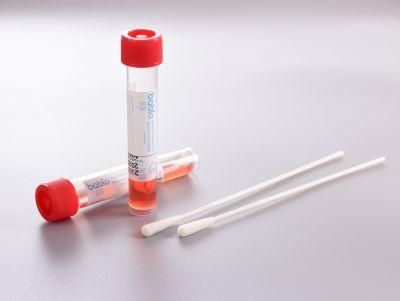
Non-Inactivated Virus Delivery Medium Factory Direct Sales
Jinan Babio Biotechnology Co., Ltd.- Quality Guarantee Period:One Year
- Group:Adult
- Logo Printing:With Logo Printing
- Product Quality Certification:CE,FDA,ISO,MSDS
- Medical Device Classification:Class I
- Transport Package:Packing
Base Info
- Model NO.:vtm
- Specification:10cm
- Trademark:babio
- Origin:China
- HS Code:3822001000
- Production Capacity:500000,Week
Description
Overview
Swabs AdvantageTest Procedure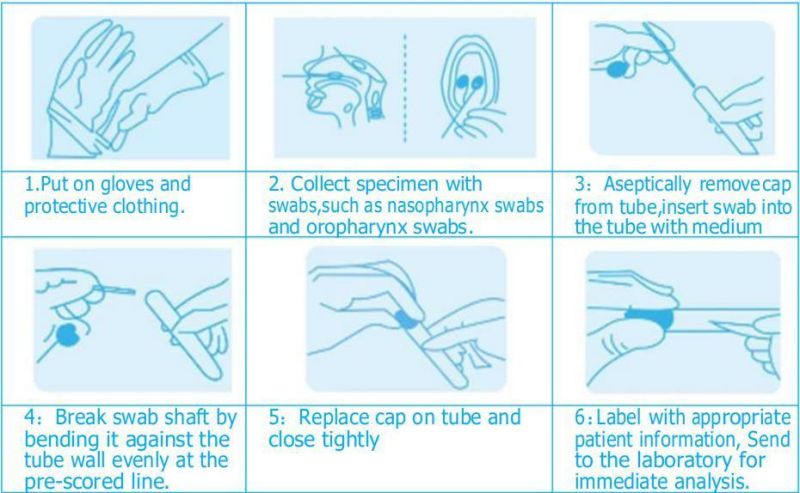 Company Profile
Company Profile Certifications
Certifications Packaging & Shipping
Packaging & Shipping
 Company Profile
Company Profile
 Certifications
Certifications
 Packaging & Shipping
Packaging & Shipping