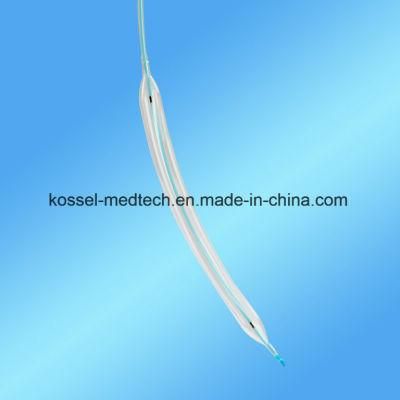
FDA Nc Ptca Balloon Dilatation Catheter Medical Device
Kossel Medtech (Suzhou) Co., Ltd.- Type:Catheter, PCI
- Material:Nylon, Pebax Nylon
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization, Ethylene Oxide Sterilization
- Quality Guarantee Period:Three Years
- Group:Adult, Adult
- Logo Printing:With Logo Printing, Both with Logo Printing and Without
Base Info
- Model NO.:NC PTCA
- Feature:Disposable, Disposable
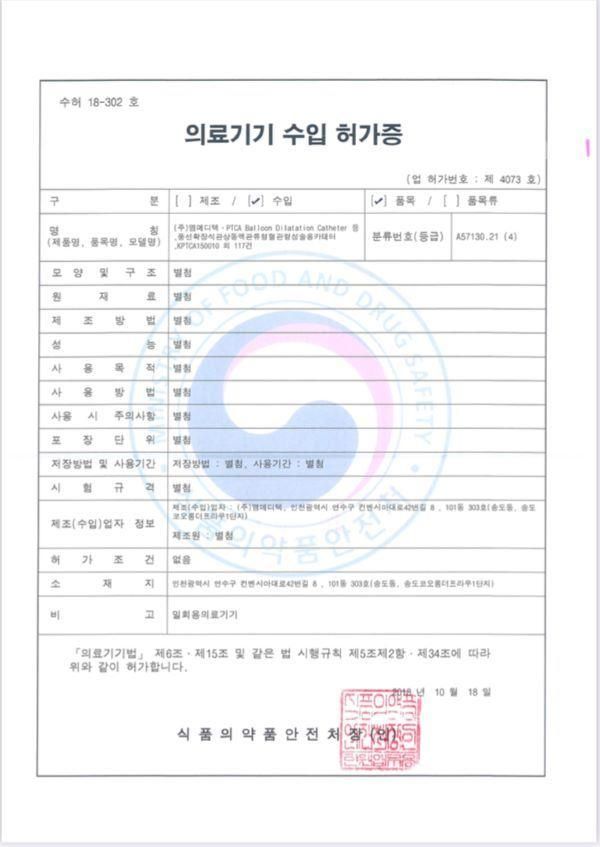
- Certification:CE, FDA, ISO13485, ISO13485
- Needle:Without Needle
- Application:Cardiovascular
- Nominal Burst Pressure:12 ATM
- Rated Burst Pressure:22ATM
- Balloon Folding:3
- Catheter Lengthcatheter Length:142cm
- Transport Package:Box or Pouch
- Specification:Diameter from 1.75mm to 5.0mm
- Trademark:OEM, Global
- Origin:Suzhou China
- HS Code:9018390000
- Production Capacity:5000PCS,Month
Description
Basic Info.
Model NO. NC PTCA Feature Disposable, Disposable Certification CE, FDA, ISO13485, ISO13485 Needle Without Needle Application Cardiovascular Nominal Burst Pressure 12 ATM Rated Burst Pressure 22ATM Balloon Folding 3 Catheter Lengthcatheter Length 142cm Transport Package Box or Pouch Specification Diameter from 1.75mm to 5.0mm Trademark OEM, Global Origin Suzhou China HS Code 9018390000 Production Capacity 5000PCS/MonthProduct Description
Company ProfileKossel Medtech (Suzhou) Co., Ltd. with the headquarter in Suzhou China, was coestablished by experts in US and domestic R&D team in 2013. Kossel Medtech focus on R&D, manufacturing and sales of Class 3 interventional cardiovascular medical devices, including products for peripheral vess,elelectrophysiology,coronary and technical service.Kossel Medtech's main clients are overseas and domestic high-end medical devices manufacturers, distributors and hospitals.



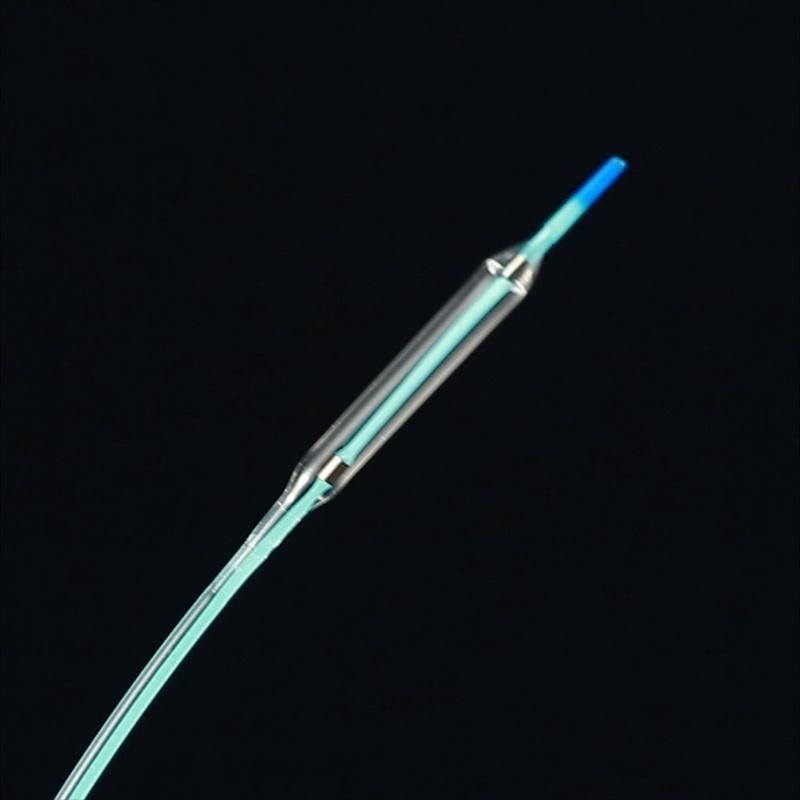
Product Features:
1. Excellent resistance to high pressure, NP-12ATM, RBP-22ATM.
2. Provide accurate concentration of expansion pressure, ultra-low compliance rate, and low inflation rate from NP to RBP.
3. Short shoulder design reduce the excess expansion at the both ends of balloon, focus mainly on the expanded lesions, and reduce the damages to normal vessel.
4. Soft tapered tip, provides smooth transition from guidewire to balloon catheter, and more advantageous to through bending blood vessels.
5. The advanced laser bonding technology ensures the balloon and the catheter smooth transition connection that makes better cross the stenosis easier.
6. Super-lubricity hydrophilic coating design can offer a superior delivery performance.
Excellent performance of rewrap after balloon expansion, more conducive to balloon withdraw.
Size Matrix
| L:mm D:mm | 6 | 8 | 9 | 10 | 12(NP) | 14 | 15 | 16 | 18 | 20 | 22(RBP) | 24 | 25 | 26 | 27 | 30 |
| 1.75 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.00 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.25 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.50 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.75 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.00 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.25 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.50 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.75 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4.00 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4.50 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 5.00 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
Production Line




Certificate