Gel and Clot Activator Tube
Hunan Runmei Gene Technology Co., Ltd.- Type:Vacuum Blood Tube & Blood Bag
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Two Years
- Group:Adult
- Logo Printing:With Logo Printing
Base Info
- Model NO.:CIH-F8B6
- Transport Package:Carton
- Specification:45*35*35
- Trademark:runmei
- Origin:China
- HS Code:2801100000
- Production Capacity:100000,Day
Description
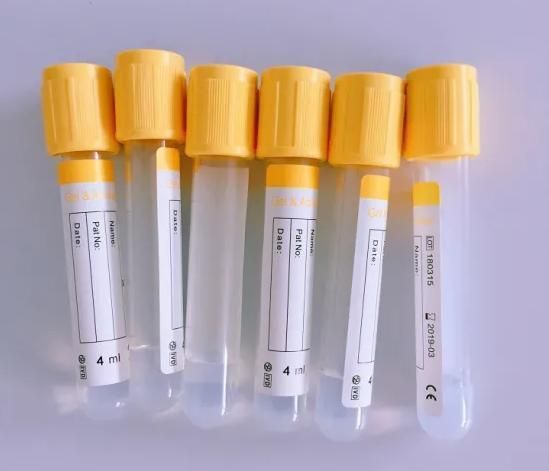
| Vacuum Blood Collection Tubes | |||||
| Gel and clot activator tube | |||||
| Cap: Yellow | |||||
| Additive: Clot Activator | |||||
| Accessory: Separating gel | |||||
| Application: Used for preparing premium serum | |||||
| for determinations in biochemistry, immunology, | |||||
| element trace, various types of virus detection and | |||||
| serology | |||||
| . | |||||
| Minimum recommended clotting time: 30 minutes. | |||||
| Notice: Suggested to centrifuge the tube in 2 hours | |||||
| after blood collection. | |||||
| Tube Material: PET/GLASS | |||||
| Label: Paper/Transparent/UV Print | |||||
| Material | Spec(mm) | Volume(ml) | Label | Shelf life(year) | Package |
| Glass | 13*75 | 3,3.5,4 | Paper label | 2 | 100/1800 |
| Glass | 13*100 | 5,6 | Paper label | 2 | 100/1200 |
| Glass | 16*100 | 7,8 | Paper label | 2 | 100/1200 |
| PET | 13*75 | 3,4 | Paper label/UV Printing | 1 | 100/1800 |
| PET | 13*100 | 5,6 | Paper label/UV Printing | 1 | 100/1800 |
| PET | 16*100 | 7,8 | Paper label/UV Printing | 1 | 100/1200 |
Company Profile
Hunan Runmei Gene Technology Co., Ltd is a high-tech enterprise dedicated to the development of the gene detection products and the construction of big data service platforms led by a team of doctors at home and abroad. Our company's strategic goal is to base on China and radiate the world, and to solve the pain points and difficulties of the industry and create value for human beings as our corporate purpose. At present, our company has completed the construction of product systems for pathogen biology fluoresence quantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biology immune colloidal gold detection kits. Our company has completed the construction of the national pathogenic biology product and service marketing network and the development of overseas detection product markets. We have a professional technical team, advanced manangement mode and perfect business operation mechanism. We are focusing on IVD detection kits. We have our own R&D team, manufacture, many of them are professional doctors who have plenty of experience in this industry. Our products have approved many certificates like ISO, SGS,CE,CFDA and so on.


