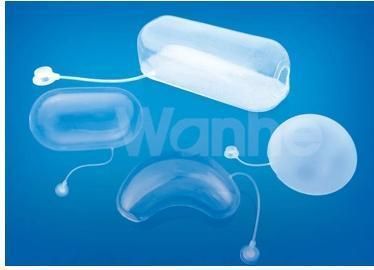
Silicone Medical Tissue Expander for Implantable Expander
Jiangsu Keqi Medical Equipment Co., Ltd.- Material:Silicone
- Feature:Reusable
- Certification:CE, FDA
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:All
Base Info
- Model NO.:c b k r
- Logo Printing:With Logo Printing
- Age Group:10-60
- FSC Certificate:ISO13485
- Apply to:Cosmetic Plastic and Reconstructive Surgery
- Target User:All
- Transport Package:Box
- Origin:China
- HS Code:40012200
- Production Capacity:50000pair Per Year
Description
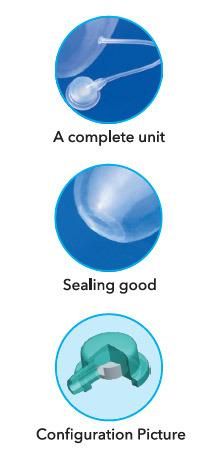
1.Expander capsule: thickness uniformity
2.Soft and harmless to the tissue
3.The direction of the expansion is synchronous
4.Safe and good sealing
5.Extra elasticity
6.Long expansion
Type:
C(Cylinder),B(ball),K(Kidney),R(Rectangl
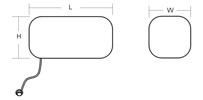
Rectangle Tissue Expander
Unit:mm
Production base



GMP standard purification area GMP
We concentrates on the improvement of product quality and the research and development of new products. With a fully equipped manufacturing base, a cleanroom in Class 10000 and 100000, advanced production and inspection devices and apparatus, We can strictly control the product quality and provide a good environment for product research, development and production. Since the products have been put into market, the market share of We has grown steadily and the products have gained the recognition of surgeons and patients in Brazil, Colombia, Russia, Taiwan and other countries and regions.