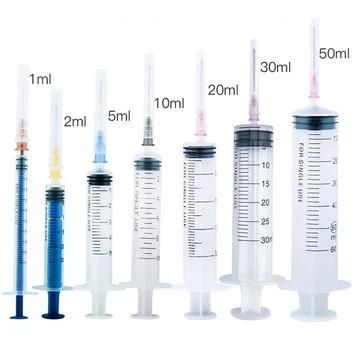
510K Registered Quality Disposable Syringe FDA
Shandong Qinkai Medical Industry Co., Ltd.- Type:Infusion Set
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Five Years
- Group:Adult
- Logo Printing:Without Logo Printing
Base Info
- Transport Package:PE
- Specification:61*38*32.5cm
- Trademark:qinkai
- Origin:China. Heze
- HS Code:9018310000
- Production Capacity:1000000pieces,Every Day
Description
Basic Info.
Transport Package PE Specification 61*38*32.5cm Trademark qinkai Origin China. Heze HS Code 9018310000 Production Capacity 1000000pieces/Every DayProduct Description
Basic Info
Model NO.
1ml 3ml 5ml, 10ml 20ml
Group
Adult
Needle
With Needle
Syringe Use
Intramuscular Injection
Disinfection
Disinfection
OEM
Available
Free Sample
Available
Sterilization
Eo Gas
Needle Type
Retractable
Trademark
OEM/ODM
Transport Package
PE Bag/Blister Pack, Junior Box, Shipping Carton
Specification
CE, ISO, GMP
Origin
China
HS Code
90183100
Product Description
Simple and easy operation;The special safety cap can avoid nurse's hands being hurt;
It can be destroyed automatically after use;
It can match different size of hypodermic needles;
Special structure, simple and convenient use;
Sizes: 1ml/CC, 3ml/CC, 5ml/CC, 10ml/CC, 20ml/CC
With or without needle
Sterile, No-toxic, No-pyrogenic, Latex-free
Material: Highly transparent medical grade PP
Package: Blister pack as unit packing, inner box, export carton
Sterilized by ETO gas, Gamma Ray Sterilizatin is Available Upon Request
Transparent Plunger is Available
Shelf life: 5 years
| Product | Safety Syringe |
| Specification | 1ml 3ml 5ml, 10ml 20ml |
| Material | Medical grade pp |
| Sterile | By EO gas |
| Type | Safety syringe |
| Package | Single in Blister package, 100pcs/box; carton package by outside |
| Advantage | After injection, the needle will retract into barrel to avoid reuse and prevent dpctors and patient being stabbed by needle |
Sterilization:
* EO gas
Specification: They are suited to hard containers and soft bags( without
Air inlet type)
Sterile, non-toxic, pyrogen free, sterilized by EOG.
Certificate: CE, ISO9002, EN46002
Our Company
Qinkai Healthcare Products Co., Ltd. is a professional company in disposable medical device area and devoted itself to researching, exploiting and manufacturing medical products of syringe, Insulin syringes, Infusion set, Burette set I. V catheter, A. V. Fistula needles, Blood collection needles, extension tube, uringe bag, surgical blades, 3-way stopcock, condoms and gloves, etc. With the principle of "professional quality, loving care for health", we have passed the ISO13485 and CEO123 certificate, our products have been exported to more than 70 countries, Such as European countries; MID east, South America&Centro America countries and Africa market, Such span, Greece, Italy, Germany, UK, USA, Mexico, Venuzuela, Chile, Brazil, Bolivia, Paraguay, Uruguay, Peru, Costa Rica, Panama, Turkey, Yemen, Jordan, Iran. UAE, South Africa, Egypt and other countres.
We are looking forward to developing long-term strategic relationships with you! !
Our Service:
We can also produce disposable syringes, disposable I.V sets, disposable insulin syringes, disposable hypodermic needles, disposable scalp veins, disposable urine bags and other medical devices.
OEM service is available

Contact person: Jack Jiang
Address:Quancheng road medical device industrial park, chengwu economic development zone, shandong province, China (qinkai group) zip code: 274200
Company Home Page: qinkaiyiliao.en.made-in-china.com
