
Disposable Trocars 3mm Bladeless Trocars 55mm Workingl Length for Endoscopic Procedures for Pediatric Patients
Guangzhou T.K Medical Instrument Co., Ltd.- Type:Surgical Supplies Materials
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:3 Years
- Group:Adult
- Logo Printing:With Logo Printing
Base Info
- Model NO.:T03-55-CT-S
- Color:Blue
- Speciality:for New Born Baby
- Transport Package:Blister Package in Carton Case
- Specification:3mm*55mm
- Trademark:TK
- Origin:China
- HS Code:90189099
- Production Capacity:200000 Pieces,Year
Description
Overview
GTK Strong Quality System
GTK Certifications
1. Q: What is the core strength of GTK Medical?
A: Our core strength is strong R&D Capability (more than 600 patents applied) and world first class quality (FDA 510K, CE, ISO13485:2016) management on products.
2. Q: What makes GTK Trocars stand out?
A: Excellent sealing performance plus excellent smooth insertion and removal of surgical instruments.
3. Q: Do you provide free samples of GTK Trocars for clinical trial use?
A: Yes. We are proud and confident to provide free samples for real clinical testing and evaluation.
4. Q: Do you have exported experiences to large medical devices company/companies?
A: Yes. We exported to more than 40 countries, most of which are based in the America and Europe.
5. Q: Do you accept factory audit prior to formal partnership?
A: Yes. We are proud and confident to accept factory audit. We passed US FDA on site aduits on 2015 and 2021.
Send me an inquiry, I will provide you with best product at the most competitive prices with warm services.
GTK Core Strength
GTK Medical devices are exported to more than 40 countries including but not limited to the UK, US, Germany, France, Italy, Spain, Belgium, Japan, South Korea etc.
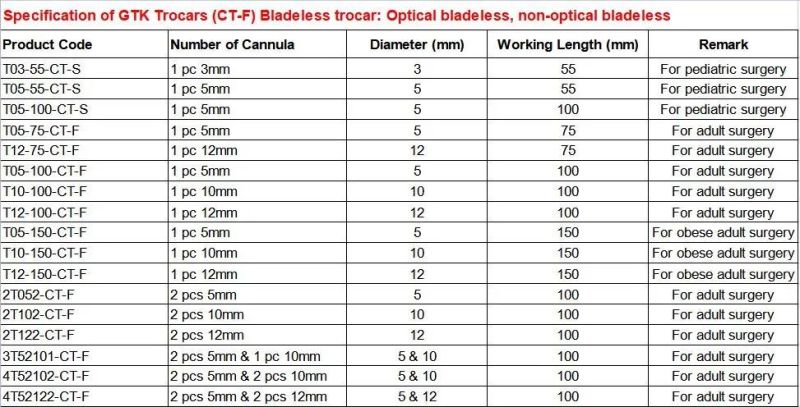
GTK Trocar detailsGTK Products Sepcifications
GTK Trocars specifcations meet the needs of all clinical demands;

GTK Certifications




Q&A on GTK Trocars;
1. Q: What is the core strength of GTK Medical?
A: Our core strength is strong R&D Capability (more than 600 patents applied) and world first class quality (FDA 510K, CE, ISO13485:2016) management on products.
2. Q: What makes GTK Trocars stand out?
A: Excellent sealing performance plus excellent smooth insertion and removal of surgical instruments.
3. Q: Do you provide free samples of GTK Trocars for clinical trial use?
A: Yes. We are proud and confident to provide free samples for real clinical testing and evaluation.
4. Q: Do you have exported experiences to large medical devices company/companies?
A: Yes. We exported to more than 40 countries, most of which are based in the America and Europe.
5. Q: Do you accept factory audit prior to formal partnership?
A: Yes. We are proud and confident to accept factory audit. We passed US FDA on site aduits on 2015 and 2021.
Send me an inquiry, I will provide you with best product at the most competitive prices with warm services.
