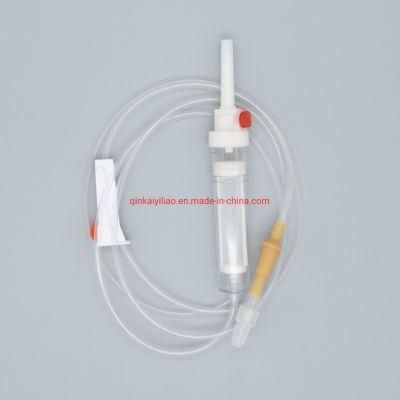
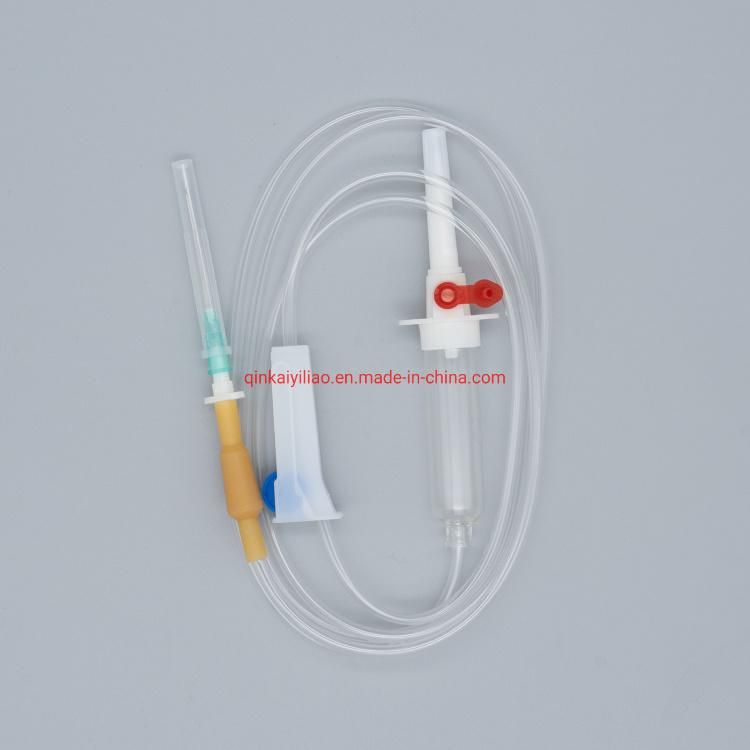
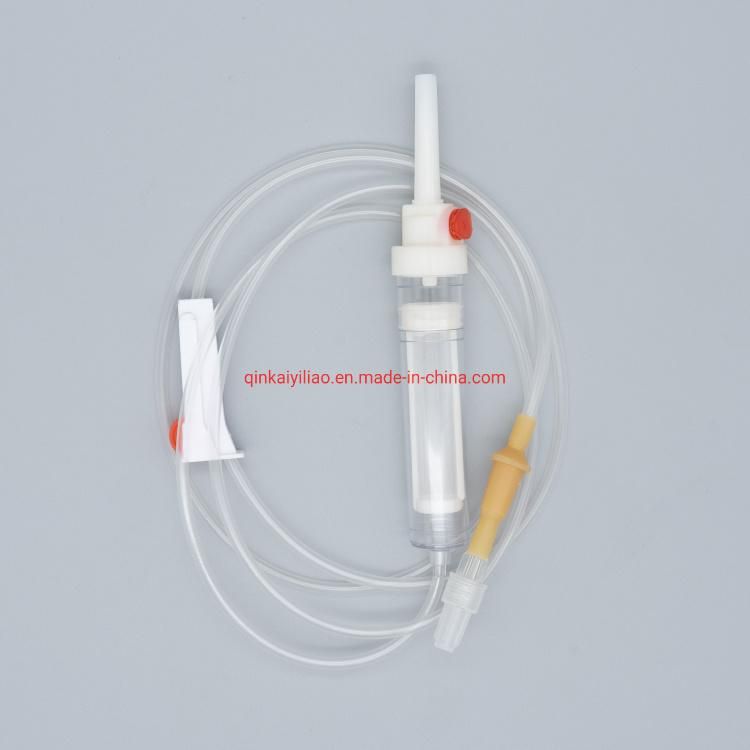
Ce/ISO Approved Medical Disposable Blood Transfusion Set
Shandong Qinkai Medical Industry Co., Ltd.- Type:Infusion Set
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Five Years
- Group:Adult
- Logo Printing:Without Logo Printing
Base Info
- Model NO.:blood
- Transport Package:PE Bag and Blister Paper
- Specification:61*38*32.5cm
- Trademark:qinkai
- Origin:China. Heze
- HS Code:9018390000
- Production Capacity:300000pieces,Every Day
Description
Basic Info.
Model NO. blood Transport Package PE Bag and Blister Paper Specification 61*38*32.5cm Trademark qinkai Origin China. Heze HS Code 9018390000 Production Capacity 300000pieces/Every DayProduct Description
OverviewQuick DetailsProperties:Medical Polymer Materials & ProductsPlace of Origin:Shandong, China
Brand Name:QINKAI
Model Number:QK7022
Type:Transfusion Apparatus
Package:Bulk/PE or blister package
Certificates:CE, ISO9001, ISO13485 and FDA on the site audit
Leadtime:Usually 30 days after samples and artwork confirmed
Payment method:L/C or T/T
Supply Ability:
10000000 Piece/Pieces per Month
Packaging & DeliveryPackaging Details
PE or Blister package or according to customers' requirements
Port
Qingdao or Shanghai Product Details
CE/ISO Approved Medical Disposable Blood Transfusion Set
Specification _________
1) For blood bags 2) 20 drops =1+0.1ml 3) Flexible drip chamber 4) Roller clamp 5) Slip or lock adapter 6) With/without Y-injection port 7) With/without vented spike 8) For gravity use 9) Sterile |
Contact person: Jack Jiang
Tel: +86 0530 6112108
Mobile phone: +86 17753067899
Address:Quancheng road medical device industrial park, chengwu economic development zone, shandong province, China (qinkai group) zip code: 274200
Company Home Page: qinkaiyiliao.en.made-in-china.com
