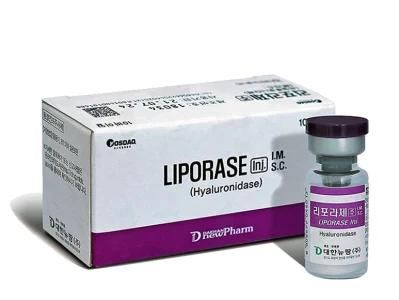
Korea Skin Care Sodium Anti Lyase Remove Ha Hyaluronic Acid Dissolving Liporase Hyaluronidase Dissolver Injection
Shijiazhuang Bogo Tech Co., Ltd.- Certification:Cfda
- Application:Face and Body
- Transport Package:Box
- Specification:1500units,vial*10vials
- Trademark:Liporase
- Origin:Korea
Base Info
- Model NO.:1500units,vial*10vials
- Production Capacity:100000,Month
Description



