-
Factory Supply The Disposable CE and ISO Approved Medical Surgery Sterile Cardiovascular Surgical Pack / Cardiovascular Pack Without Surgical Gown
![Factory Supply The Disposable CE and ISO Approved Medical Surgery Sterile Cardiovascular Surgical Pack / Cardiovascular Pack Without Surgical Gown]()
Basic Info. Model NO. JKG-006
-
Clinic Disposable Hospital Drapes Disposable Extremity Surgery Medical Drapes

Drape Dimensions:200*300cmLiquid Collection Pouch Size90*120cmHole Size5cmPackingIndividual packed, 1Pc/Pouch,25Pouch/Ctn,Description:Extra length for coverage where it's neededConformable, 2.5 in. diameter fenestration fits around patient's limbAbsorbent Fabric ReinforcementConvenient, built-in arm
-
Customized Disposable Sterile Surgical Drape/Surgical Side Drape/Adhesive Drape
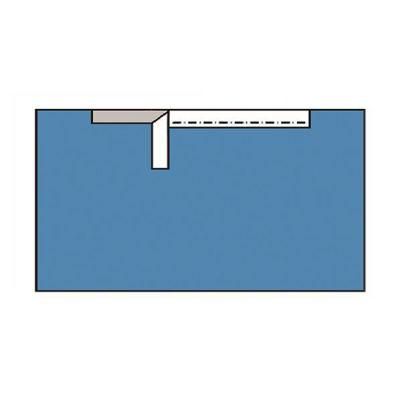
ISO 13485, CE
-
Medical Supply of Eye Surgery Set Ophthalmic Pack
Quantity per PackComponent ListingsComponent Size1pcInstrument Table Cover150x200cm1pcOphthalmic Drape100x130cm2pcsHand Towel40x40cm2pcsStandard Surgical GownL1pcWrapper100x100cm1. Reinforced Instrument Table Cover: Used to wrap all the accessories in the surgical kit. After the surgical kit is open
-
Type 4 Protective Coveralls with En14126 and En14605, Medical Protective Clothing

Basic Info. Model NO. S/M/L/XL/XXL/XXL
-
Reinforced Surgical Gown for Doctor Workwear

Basic Info. Model NO. JKG-023
-
Three-Part /3 Part Hos Medical Sterile Hospital Plastic Disposable Syringe 3ml with Needle Luer Lock Slip

SIZEby EO gas, sterile, non-toxic, non-pyrogen, single use only.Consist ofBarrel, Plunger, Standard Piston or Latex Free Piston, Cannula, Needle hub, Needle protector, Lubricant, Graduation, Plastic bag.Material:BarrelMaterial:medicalandhightransparentPPwithPlungerstoppedring.Standard:1ml 3ml5ml10ml
-
Factory Supply The Disposable CE and ISO Approved Medical Surgery Non-Woven Level 2/Level 3 Surgical Gown

Size of the gownLength and Width 1Length and Width 2S110*140cm115*152cmM115*145cm130*152cmL127*150cm140*160cmXL135*150cm150*176cmXXL140*155cm155*176cmXXXL145*160cm160*180cmCompetitive Advantage:1. Absolutely dustless clean workshop for clothing making.2. We stick to strict quality inspection standar